Which of the Following Nuclei Would Be the Least Stable
Stable lightweight nuclei start the process. CNucleus A is the most stable and nucleus D is not stable.
21 1 Nuclear Structure And Stability Chemistry
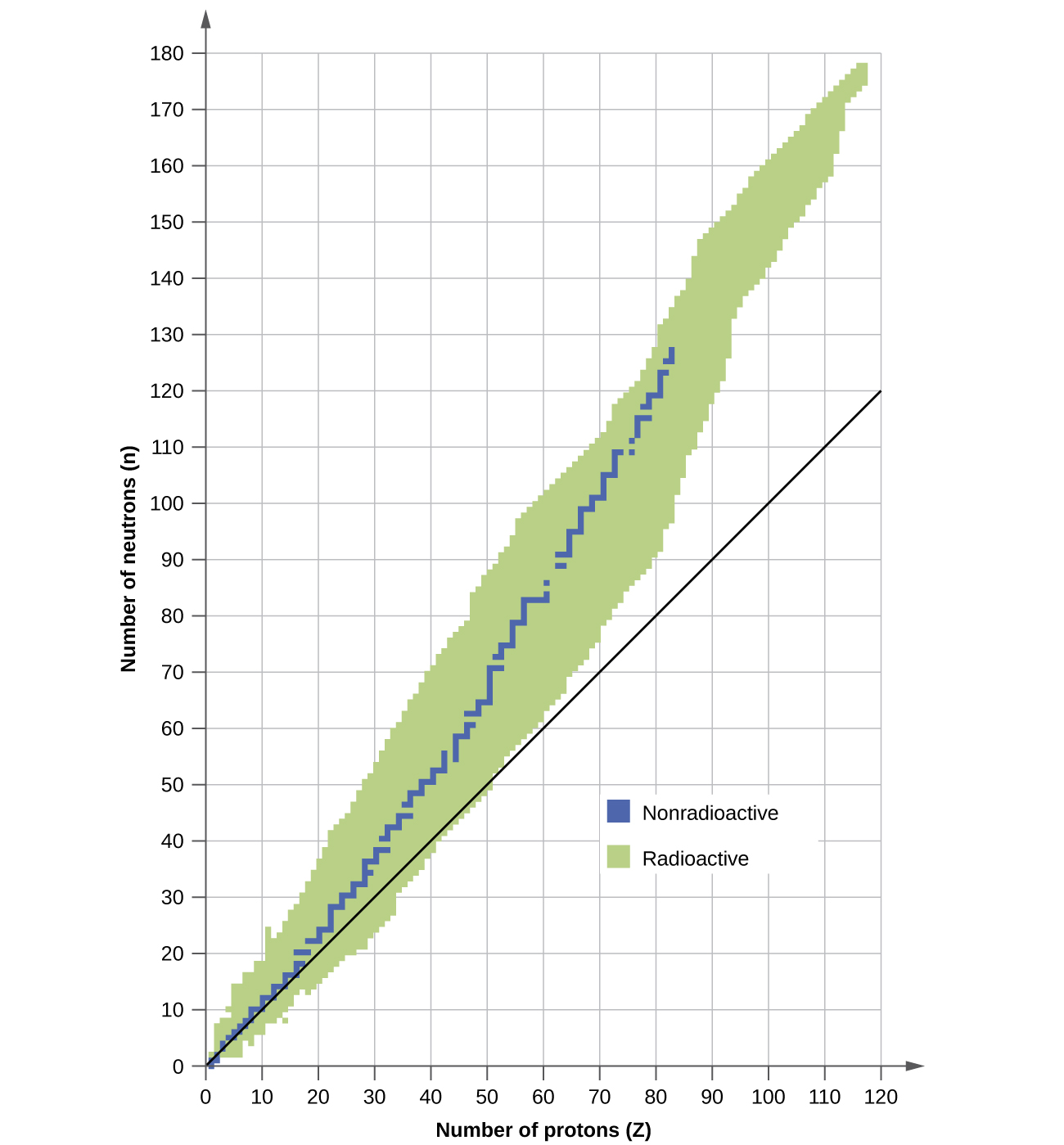
The nuclei He-4 O-16 and Pb-208 82 protons and 126 neutrons that contain magic numbers of both neutrons and protons are particularly stable.
. On the other hand nuclei with an odd number of protons and neutrons are mostly unstable. Representations of three nuclei with neutrons gray and protons purple are shown below. Iron has the maximum average binding energy 879 MeV and thus its nucleus is thermodynamically most stable.
The correct answer would be option D. Which statement is true regarding the stability of these nuclei. All elements to element 98 are found in nature.
Nuclei having binding energy per nucleon very near to 8 MeV are more or less stable. Therefore lead is considered the heaviest stable element. In contrast the decay of the excited nuclear.
The average binding energy for most of the nuclei is in the vicinity of 8 MeV. Nuclides containing even numbers of both protons and neutrons are most stable and this. 10 protons 12 neutrons because the closer the neutrons to the number of protons the more stable.
Assume they all have stable nuclei. BNucleus C is stable whereas nuclei A B and D are not. The time interval during which a nucleus has a 50 probability of decaying is its.
Some artificial radioactive isotopes can be prepared by bombarding stable nuclei with. The stable elements at the end of the decay series all have a magic number of neutrons or protons. Which of the following processes produces nuclei of lower mass than the reactants.
10 protons 12 neutrons. DNuclei A and B are stable but nucleus B is more stable than nucleus A. The rest mass of a stable nucleus is less than the sum of the rest masses of its separated nucleons C In nuclear fission energy is released by fusing two nuclei of medium mass approximately 100 amu.
C The curve has certain peaks indicating that certain nuclei like C He 12 6 4 2 and O 16 8 are much more stable than the nuclei in their vicinity. For example helium-4 is among the most abundant and stable nuclei in the universe. See the related Wikipedia linkLarger atomic nuclei.
Which of the following nuclei would be the most stable. Lighter nuclei have np ratio around 1. The most stable nuclei are iron and nickel and that is due to the binding energy per nucleon being greatest in that size of nucleus.
10 protons 14 neutrons c. All stable isotopes stable by observation not theory are the ground states of nuclei with the exception of tantalum-180m which is a nuclear isomer or excited state. 4 B e 1 0 nuclei is unstable due to high np ratio which is 4 1 0.
Nucleus 1 is stable. Lighter nuclei have np ratio around 1. Correct answer to the question Which of the following nuclei would be most stable.
ANucleus D is the most stable and nucleus A is the least stable. The ground state of this particular nucleus tantalum-180 is radioactive with a comparatively short half-life of 8 hours. Which of the following lists ranks nuclear radiation from most massive to least massive.
Heavy nuclei with an even number of protons and an even number of neutrons are due to Pauli exclusion principle very stable thanks to the occurrence of paired spin. When a nucleus in an excited state decays to the ground state the particle emitted is. Was this answer helpful.
10 protons 15 neutrons d. B The light nuclei with A 20 are least stable. All travel at the same speed.
Solutions for Chapter 24 Problem 148P. For each of the 80 stable elements the number of stable isotopes is given. For example tin has 10 such stable isotopes.
10 protons 13 neutrons b. There are 80 elements with at least one stable isotope but 114 to 118 chemical elements are known. The isotopes with intermediate mass numbers 40 to 100 are most stable.
The mass of an atom of carbon-12 Z 6 is less than the mass of 6 protons and 6 neutrons because of. As you go to heavier nuclei like uranium for instance thenucleus gets less stable. Which of the following noble gases would behave the LEAST like an ideal gas.
Nuclides containing odd numbers of both protons and neutrons are the least stable and this means more radioactive.

Nuclear Fission Fundamentals Of The Fission Process Britannica
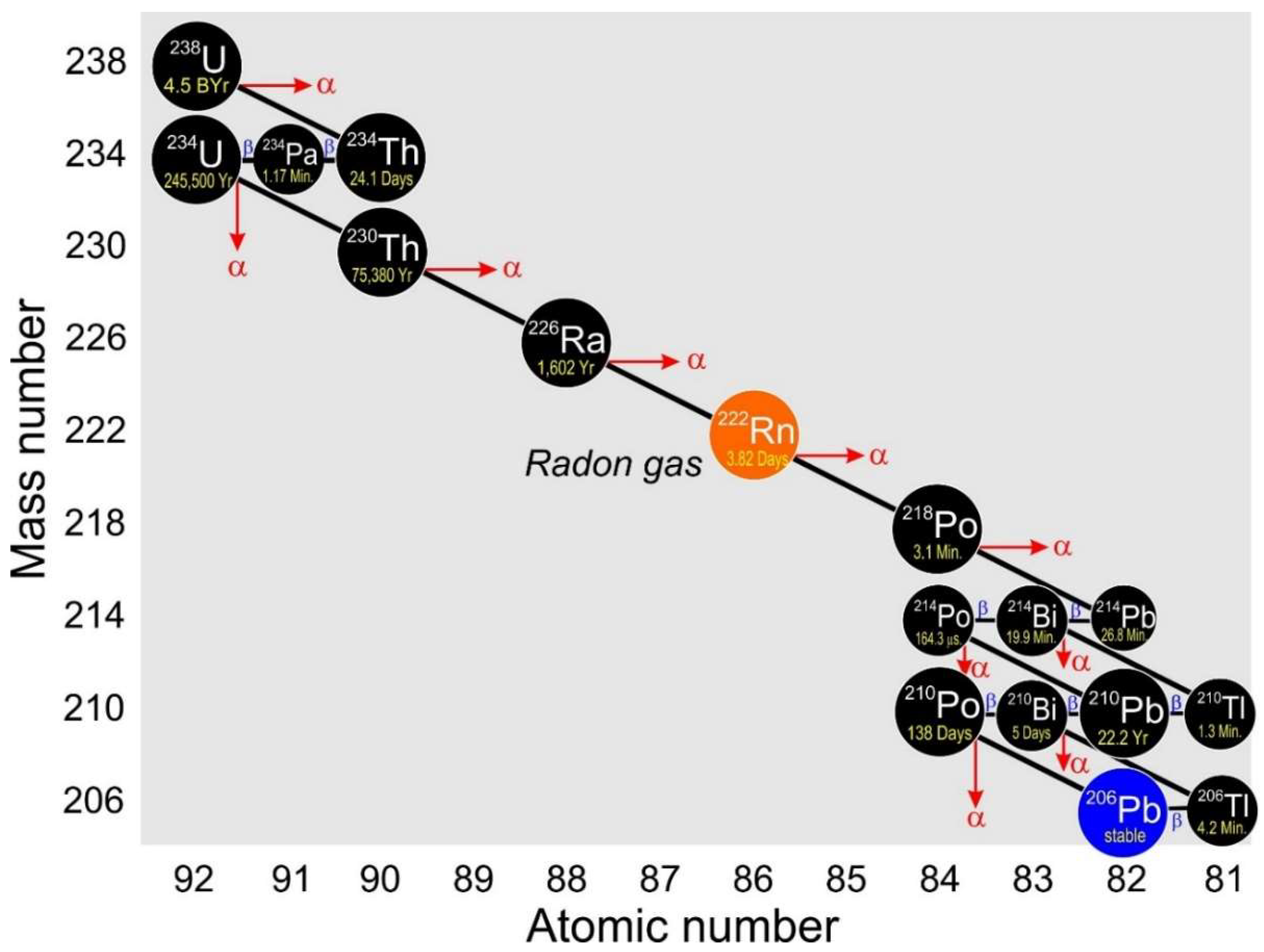
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html

Minerals Free Full Text How Can Additives Control The Early Stages Of Mineralisation Html
No comments for "Which of the Following Nuclei Would Be the Least Stable"
Post a Comment